Background: Primary central nervous system lymphoma (PCNSL) is a rare form of non-Hodgkin lymphoma with two-year overall survival (OS) rates of 66-70% reported in prospective clinical trials. Induction therapy with high-dose methotrexate (HD-MTX) followed by consolidation has become the mainstay of treatment, however, there are few contemporary and robust prognostication indices widely available for clinical use. Some of the most used indices were developed prior to the routine use of rituximab and include the International Extranodal Lymphoma Study Group (IELSG; 1980-1999), Memorial Sloan-Kettering Cancer Center (MSKCC; 1983-2003), Nottingham/Barcelona (NB; 1986-2001), and Taipei Score (TS; 2003-2015). Herein, we describe a contemporary comparison of clinical prognostication indices using a single-center cohort of PCNSL patients treated at the Mayo Clinic.
Methods: Patients with a diagnosis of PCNSL who received HD-MTX as part of induction therapy at Mayo Clinic between October 2010 and June 2022 were identified and retrospectively reviewed. Patients with prior or concurrent systemic lymphoma were excluded. Primary endpoints were progression-free survival (PFS) defined as time from diagnosis to relapse, progression, or death due to any cause; and OS defined as time from diagnosis to death due to any cause. PFS and OS were evaluated using Kaplan-Meier curves and compared by risk scores using a log-rank test. The association between baseline characteristics and survival outcome were assessed in univariate Cox regression models. Any characteristics showing significant (p-value <0.05) association with outcome were combined using a multivariate Cox regression model. All characteristics that maintained significant association with outcome were combined into a new prognostic model. We compared the performance of each prognostic index model using Harrell's C (HC).
Results: A total of 148 patients were identified (50.7% female) with median age at diagnosis of 66 years (range 29-85), with 48 patients (32.4%) age >70 (Table 1). Most had multifocal disease (60.1%) and deep brain involvement (67.6%) at diagnosis with elevated CSF protein (58.1%) but no documented CSF involvement (93.9%). In total, 117 patients (79.1%) received methotrexate, rituximab, and temozolomide (MRT) induction therapy, and the remaining 31 patients (20.9%) received methotrexate and rituximab (MR) induction therapy with various consolidation strategies. The median follow-up for all patients was 4.5 years and the two-year OS was 79% (95% CI: 73-86%).
This cohort was stratified using previously published prognostication indices (Table 1). Upon analysis, PFS was not accurately predicted using the IELSG (score 4-5: HR 0.7 (CI 0.3-2.0); HC 0.6), MSKCC (3: 1.3 (0.4-4.0); 0.5), NB (3: 0.9 (0.4-2.5); 0.6), or TS (3: 0.9 (0.1-7.2); 0.5) prognostication indices. Furthermore, OS was not accurately predicted using the IELSG (4-5: 1.5 (0.6-4.0); 0.6), MSKCC (3: 1.2 (0.5-3.2); 0.6), NB (3: 1.2 (0.5-3.2); 0.6), or TS (3: 1.3 (0.2-9.8); 0.6) prognostication indices.
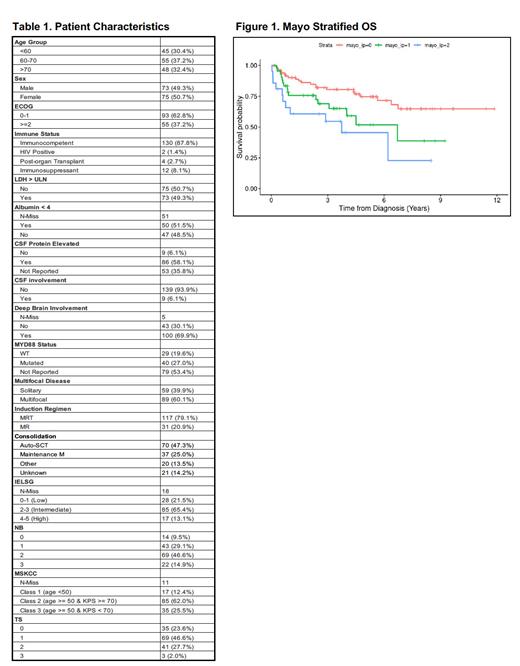
Upon multivariate analysis, no variables listed in Table 1 were associated with PFS within this cohort. The two variables predicting a worsened OS were an ECOG performance status of 2 or more (1.9 (1.01-3.6)) and an MR induction regimen instead of MRT (2.1 (1.05-4.3)) (Figure 1). The two-year OS with no risk factors was 86.1% (95% CI: 78.8-94.1%), one risk factor was 75.7% (95% CI: 63.6-90.1%), and two risk factors was 61% (95% CI: 43-86%) (Figure 1).
Conclusions: This study reports overall improved outcomes in patients with PCNSL compared to prior prospective and retrospective cohorts, likely in part due to treatment advances and supportive care strategies. This single-center, retrospective cohort of rituximab-exposed patients with PCNSL were not well prognosticated using previously published indices, indicating that further study and more sophisticated approaches to prognostication are warranted. Although multivariate analysis did not demonstrate any variables associated with PFS, it did demonstrate an inferior OS with a performance score of 2 or more and in patients treated with MR rather than MRT.
Disclosures
Witzig:Salarius Pharma: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Research Funding; Kura Oncology: Research Funding; ADC: Membership on an entity's Board of Directors or advisory committees. Paludo:Biofourmis: Research Funding; Karyopharm: Research Funding; AbbVie: Consultancy. Habermann:Genentech: Research Funding; BMS: Research Funding; sorrento: Research Funding. Nowakowski:Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Fate Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bantam Pharmaceutical LLC: Consultancy; MorphoSys: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene Corporation: Consultancy; Genentech: Consultancy; F Hoffmann-La Roche Limited: Consultancy; Debiopharm: Consultancy; MEI Pharma: Consultancy; TG Therapeutics: Consultancy; Kymera Therapeutics: Consultancy; Kite Pharma: Consultancy; Blueprint Medicines: Consultancy; ADC Therapeutics: Consultancy; Abbvie: Consultancy; Curis: Consultancy; Ryvu Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Karyopharm Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Seagen: Consultancy; Selvita Inc: Consultancy; Zai Lab Limited: Consultancy; Incyte: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal